7th European Conference on Rare Diseases & Orphan Products 8-10 May 2014 – Berlin Germany
By Danijela Szili and Thomas Bertrand.
The ECRD conference was organised by Eurordis and co-organised by DIA. It gathered more than 750 stakeholders including patient representatives, health professionals, scientists, industry, policy makers, regulators and payers from 40 countries.
The first day of the conference was dedicated to The Eurordis membership meeting with the General Assembly, followed by parallel sessions on “Learning from each other” and “Patient advocates capacity building workshops”. In the first session we could learn, for instance, how to set up a crowd-funding project in an effective way, while in the second session we were interested in learning how patient advocacy could improve access to orphan medicinal products.
Yann Le Cam gave an overview of EURORDIS advocacy proposals to develop more Rare Disease (RD) therapies and improve patient’s access, Francois Houyez informed us about the changes on HTA (Health Technology Assessment) in Europe for RD and Pauline Evers talked about adaptive licensing applied to RD.
Adaptive licensing is part of EMA (European Medicines Agency)’s efforts to improve timely patient access to medicines and builds on regulatory processes already in place within the existing legal framework. These include scientific advice, compassionate use, the conditional marketing authorisation mechanism (for medicines addressing life-threatening conditions), patient registries, pharmacovigilance tools and development of the risk-management plan for each medicine. Developing the adaptive licensing approach requires cooperation between a wide range of stakeholders. These include the organisations that influence patient access to medicines such as the EMA and other medicines regulators, the pharmaceutical industry, HTA bodies and organisations issuing clinical treatment guidelines, as well as patient and consumer organisations, healthcare professionals, researchers and academics.
In March 2014, the EMA began inviting companies to participate in a pilot project on adaptive licensing, and published a framework to guide discussions on individual pilot studies.
EURORDIS Membership Meeting presentations including must read Activity Report 2013 can be found on the EURORDIS website:
http://www.eurordis.org/content/eurordis-membership-meeting-2014-presentations
The General Assembly put the spotlight on the different EURORDIS Volunteers who play an active and vital role in the success of EURORDIS. To properly recognize the important contribution of volunteers, a new EURORDIS website section has been created and the Charter of the EURORDIS Volunteers was presented and adopted.
From 9-10 May the ECRD sessions were divided onto 6 main themes:
1- Improving Healthcare Services
2- Knowledge Generation and Dissemination
3- Research from Discovery Patients
4- State of the Art and Innovative Practices in Orphan Products
5- Emerging Concepts and Future Policies for Rare Disease Therapies
6- Beyond Medical Care
We were particularly interested in the 1st theme focused on Centres of Expertise (CoE) and European Reference Networks (ERNS).
The first Friday session was dedicated to models and practical examples of Centres of Expertise. This session looked at the interpretation of the concept of Centres of Expertise in different countries. The role of Centres of Expertise in healthcare delivery for rare diseases is highlighted in the EUCERD recommendations, and a pillar of the national planning process. In addition, it is envisaged that Centres of Expertise will play a major role in the future European Reference Networks. In this session we explored the experience of two different models for Centres of Expertise- one focussed on a single disease and one with a much broader remit. Then we learned about the operation of CoE as part of an advanced National Plan for Rare Diseases with local networks in France.
Leena Bruckner-Tuderman, Professor and Chair of the Department of Dermatology, University Medical Center Albert-Ludwigs, University of Freiburg, Germany, presented a specialised centre for epidermolysis bullosa (EB) in Freiburg. After a description of this skin disease, Leena explained how the centre was born. It all started in a personal research interest, then in 2002 came an opportunity to concretise this project through a competitive research programme on « Networks for Rare Diseases » funded by the German Federal Ministry of Education and Research. The EB-network was, in 2003, composed of clinics, hospitals, and research laboratories in Germany gathering clinicians, physician scientists and basic scientists.
The department of Dermatology, University Medical Centre Freiburg, hosted the central office of the German EB-Network with one single coordinating person having a 24hrs availability and response time. The centre had a standardised clinical practice. From 2004 to 2013, the EB-Centre Freiburg received 1139 consultations and received diagnostic samples from 36 countries worldwide. The centre also hosts a Molecular Dermatology lab where basic science and clinical studies are performed.
http://www.rare-diseases.eu/wp-content/uploads/2014/05/0101_Leena_BRUCKNER-TUDERMAN.pdf
John Rosendahl Ostergaard, Clinical Professor, Centre of Rare Diseases, Aarhus University Hospital, Denmark, presented a talk « Perspective of a Centre of Expertise with a broader remit than one rare disease ». John explained that 85% of patients with a rare disease have symptoms within the initial five years of life. The problem is that at childhood, the medical specialty is based on age not organs, while in adulthood the specialities are based on organs. Rare diseases (RD) are crossing these borders. In Denmark there are 2 centres of expertise for rare diseases since 1996. In 2001 the Danish Health and medicine authority submitted a statement for rare diseases. There were specific plans for 11 RD, the others needed to be coordinated by highly specialised experts in unspecialised medical centres. In 2010 (still in progress), a national plan for specialised medical care is established. John presented the CoE in Aarhus which hosts more than 1,200 patients with more than 100 different diagnoses. The core (coordinating) staff is composed of 2 nurses, 2 secretaries, 3 consultants and 1 paediatrician (still in training). The core activities of Aarhus centre include diagnosis, treatment, coordination (inside the centre and outside with other hospitals), counselling and guidance.
John also mentioned the challenges:
– It is impossible to be expert in >100 diagnoses;
– The centre hosts more than 300 new patients each year;
– The transition from childhood to adulthood;
– RD do not respect the borders for either age nor organ;
– Patients cannot easily go on their own investigation, they need to see a doctor first, and doctors are not all aware of CoE.
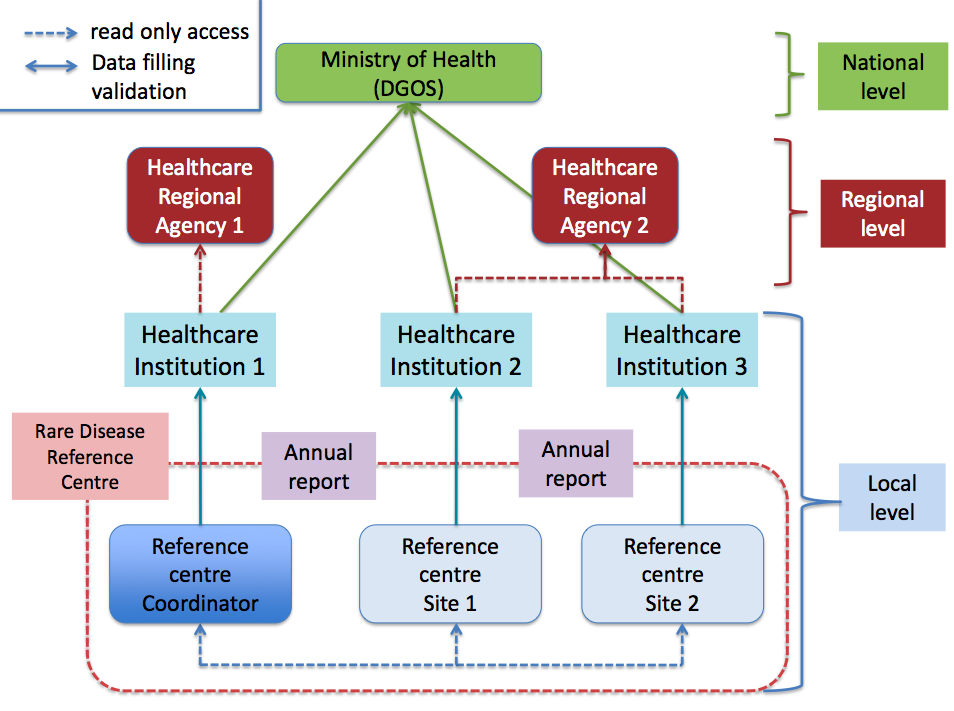
Professor Pierre Sarda, Département de Génétique Médicale, Hôpital Arnaud de Villeneuve, France, gave a presentation entitled « French Centre that demonstrates how they work in a broader healthcare system / how they interact with local networks ». The Montpellier Reference Centre for developmental and malformation disorders manages disabled people with multiple and combined disabilities: psychomotor delay in the young child, intellectual deficiency, associated with behavioural disorders in over 50% of cases, failure to thrive, organ malformations, sensory anomalies and other disorders. These disorders concern close to 3% of the population.
The Montpellier Reference Centre for developmental and malformation disorders was created in 2005. One of the missions of the Reference Centre is to facilitate diagnosis and define the strategy for the various aspects of management: therapeutic, psychological, social and educational support, as well as to animate and coordinate the network of health and medico-social correspondents. In 2009, on the request of the local antenna of the Rare Disorder Alliance, a study was done in the Languedoc-Roussillon region. The subject of this study was ” Expectations and needs of patients and families who have a rare disease”. This study highlighted the difficulties encountered by patients and families affected by a rare disease in the region. The creation of a regional health network for the needs of patients with developmental anomalies followed. The creation of this network was approved by the administrative authorities in 2009. The main mission of the network was to contribute to the implementation of a coordinated network for the local management and support of children and adults with a developmental anomaly. The network is steered by a board of directors composed of various members:
– A college of professionals: private or public doctors and paramedical specialists, representatives from medico-social institutions and national education;
– A college of associations and family group representatives.
For the regional territory 2,300 professionals are listed on the website. 440 professionals participated in training sessions (340 in 2012) and 238 patients or family members participated (236 in 2012).
http://www.rare-diseases.eu/wp-content/uploads/2014/05/0101_Pierre__SARDA.pdf
The second Friday session was dedicated to designation and evaluation of Centres of Expertise and in particular best practice examples. CoE are key components of healthcare planning for patients with rare diseases, to provide improved capability for diagnosis and specialised management. In addition, centres of expertise will be core members of the European Reference networks for Rare Diseases as planned under the Cross Border Health Care Directive.
Sabine Sarnaki, Coordinator of expert centre on anorectal and rare pelvic malformations, Hôpital Necker Enfants Malades, APHP and Paris Descartes University, France, gave a talk on the « Evaluation of Centres of Expertise: The French Experience since 2009 ». Sabine reminded that in France, after the 1st French national Rare Disease Plan 2004-2007, there were 131 centres of expertise and 501 centres of competence, which was way too much to coordinate in a useful way.
The aims of the 2nd French National Rare Disease Plan (PNMR2 – Plan National Maladies Rares 2) 2010-2014 were:
– The Simplification of the evaluation process: simplification and clarification (avoid redundancy);
– More discrimination between centres and among the sites constituting the centre of expertise allowing global analysis;
– Improve the reactivity of the steering committee to take into account new actors in the rare disease landscape and to improve the process of certification;
– Take into account the new objectives of the PNMR2: healthcare pathway, Europe, research…
The elaboration of simplified tender specifications with 7 chapters (instead 6) and the 3 usual parts (anticipation-implementation- evaluation) with the following chapters:
1. Improvement of the quality of healthcare;
2. Expertise;
3. Referral;
4. Research;
5. Education and information;
6. Coordination;
7. Position of the patient, family and associations.
http://www.rare-diseases.eu/wp-content/uploads/2014/05/0102_Sabine_SARNACKI.pdf
Edmund Jessop, medical advisor at NHS England, National Health Service UK, focussed his talk on « Quality criteria; Outcome Measures ».
The National Health Service (NHS) in UK is based on specialised services not rare diseases (except a very few like haemophilia and cystic fibrosis) with 75 “clinical reference groups” building the monitoring programme.
The NHS outcome framework includes Clinical (Mortality, Quality of Life and Recovery), Safety (The “never event”) and the Patient Experience. NHS England uses Clinical Dashboards permitting the selection a small number of items for routine monitoring, with professional agreement and is pilot for 15 – 20 services and such, each centre can compare how they achieve against all others. The Dashboard also includes items such as the number of patients for each disease, the research output and staffing.
http://www.rare-diseases.eu/wp-content/uploads/2014/05/0102_Edmund_Jessop.pdf
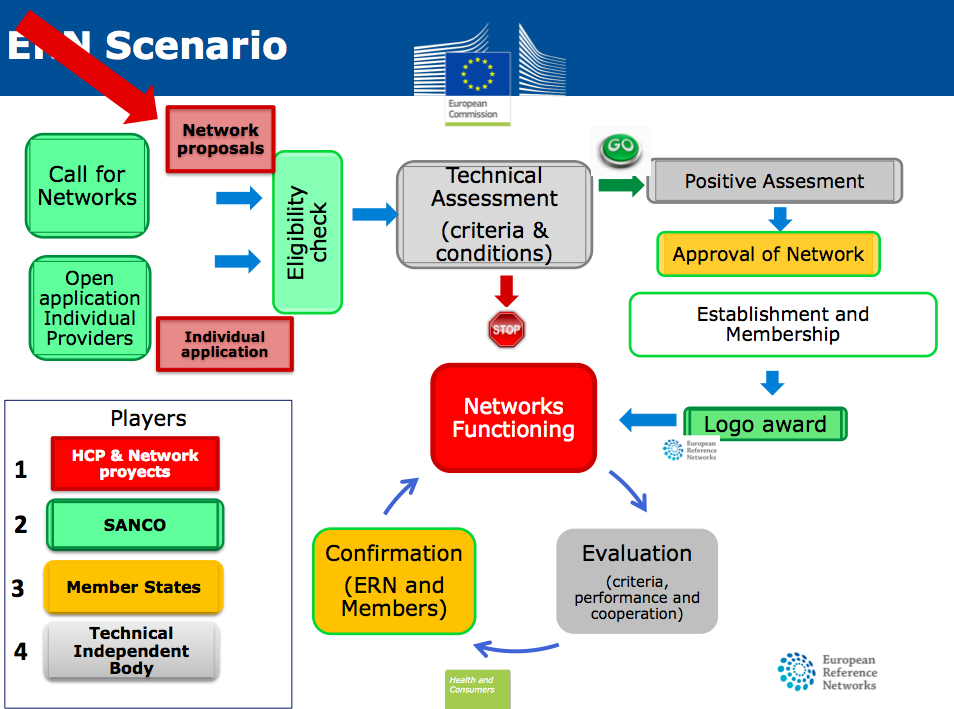
Enrique Terol, Policy Officer, DG SANCO, Health and Consumers Unit, European Commission, EU, gave a very detailed talk on the « Designation Process: How centres are selected; how to evaluate centres for rare diseases. How they plan to evaluate in the future. ». Enrique explained that the Directive 2011/24/EU of patients’ rights in cross-border healthcare article 12 concerns the cooperation between Member States. This is where the idea of European Reference Networks comes from. These are Networks of healthcare providers, which aim at improving access to highly specialised centres (e.g. centres specialised in Rare Diseases healthcare). After a full description of the implementation process, Enrique communicated the timelines for ERNS implementation: 10 March 2014 the text was adopted, 17 May the text was publish in the Official Journal, from 27 May the deployment process can start.
http://www.rarediseases.eu/wp-content/uploads/2014/05/0102_Enrique_TEROL2.pdf
The Saturday session continued to be dedicated to European Reference Networks (ERNS). In 2003/2004, the high level European reflection process on cross-border healthcare initiated, amongst others, a discussion regarding the pan-European establishment and designation of highly specialised medical centres (so-called “Centres of Expertise”) and their interlinking in European Reference Networks (ERNS).
Ten years later, the preparatory work for this concept has been largely finished with the implementation of the Cross-border Healthcare Directive (in October 2013) and the adoption and entering into force of accompanying legal acts (in May 2014). At the end of this year, the first call for proposals for ERNS will be published. The Saturday session highlighted the current practices in the member states, as well as the details and implementation strategy of the ERNS concept, followed by a panel discussion looking at existing experiences with the establishment and management of European networks in the fields of healthcare and research, as well as possible future strategies to ensure sustainability of ERNS once established.
Willy Palm, Dissemination Development Officer, European Observatory on Health Systems and Policies, Belgium, gave a talk entitled « Observatory Study – Building European Reference Networks in Health Care ». Willy introduced the concepts of « soft-core » and « hard-core » laws in legislation. For instance, the creation of Centres of Expertise (CoE) is a 2009 European Council recommendation and at the time CoE were individually managed by Member States (MS) in their national plans (“soft-core” laws). The European Reference Networks (ERNS) is a 2011 cross-border healthcare directive and supersedes CoE with the implementation of important regulations (“hard-core” laws). Willy then retraced the evolution of concepts throughout time:
Centres of Excellence -> Centres of Reference -> Centres of Expertise -> Networks of Centres of Reference -> Reference Networks.
The goals being sharing resources (cost effectiveness), concentrating cases (safety) and integrating/standardizing care (quality).
http://www.rare-diseases.eu/wp-content/uploads/2014/05/0103_Willy_PALM.pdf
During the roundtable discussion, Kate Bushby, Professor of Neuromuscular Genetics, MRC Centre for Neuromuscular Diseases, Institute of Genetic Medicine, International Centre for Life, Newcastle Upon Tyne Hospital, UK, explained that, in her experience, the trust was the most important and large number of face to face meetings was needed. These meetings were financed by what she called « glue-money » necessary to stick pieces of the network altogether. She also mentioned that, in the UK, 1/10 of CoE closes because they don’t reach the quality criteria anymore as the personnel leaves and that is the reason why Kate insisted on the fact that there should be at least 2 people doing the same job at the same time. Kate also insisted that the paperwork should not be to overwhelming for setting up ERNS because some specialised doctors might give up: not only patients are rare, the experts are even more rare.
Gabi Pohla-Gubo, Head of Epidermolysis Bullosa (EB) Academy, General Hospital Salzburg/Salzburger Landesklinikum (SALK), Paracelsus Medical University Salzburg (PMU), Austria, also mentioned that amongst the big challenges are:
– The paperwork (It took 3 years to get permission from the Austrian Health committee to build the « specialised house » with 3 units at the University);
– The data protection (inside and outside the country);
– The collaboration is not easy (the yearly update factsheet requested to all partners does not easily come back or is often filled improperly).
The last session, How Centres of Expertise should/could interface with social services, highlighted the importance of including social services and social workers in the work of Centres of Expertise. The participation of patients and their families would not be possible without help of the social services (provide information about reimbursement options, special assistance for traveling…)
The ECRD conference ended with the 3 poster prizes offered by the steering committee:
se-atlas: Cartographic Representation of Experts on Rare Diseases – Holger Storf, Tobias Hartz, Wulf Pfeiffer, Kathrin Rommel, Mareike Derks, Elisabeth Nyoungui, Jörg Schmidtke, Holm Graessner, Mirjam Knoell, Thomas Wagner, Frank Ückert
Integration of Rare Diseases into Social Services – R. Castro, D. Dan
Prenatal therapy in developmental disorders: drug targeting via intra-amniotic injection to treat X-linked hypohidrotic ectodermal dysplasia – Katharina Hermes,Pascal Schneider, Peter Krieg, AnhThu Dang, Kenneth Huttner, Holm Schneider
The ECRD full program can be found here:
http://www.rare-diseases.eu/wp-content/uploads/2013/08/ECRD2014Berlin_Final-programme.pdf
The speakers’ presentations can be found here:
http://www.rare-diseases.eu/programme/speakers-presentations-ecrd-2014/
Some photos here:
https://www.flickr.com/photos/eurordis/sets/72157644254307577/
Other resources:
http://www.eurordis.org/news/ecrd-2014-berlin-putting-rare-disease-puzzle-place